Objectives:
1. To isolate caffeine from tea by solid-liquid and liquid-liquid extraction
2. To purify the product by sublimation
Introduction:
The components of tea leave include protein, polysaccharide, pigments and amino acids (3-5%), caffeine (2-3.5%), polyphenols (catechin and tannin), carbohydrate, gallic acid, ash and small amount of saponins. In this experiment, both solid-liquid extraction and liquid-liquid extraction methods are being used to isolate caffeine from tea leaves. Solid-liquid extraction is used to separate the components that present in the tea leaves. Liquid-liquid extraction is used to isolate caffeine alone from the other components of tea leave.
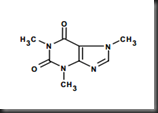
In solid-liquid extraction, tannin, pigment, glucose, amino acid, protein and saponin will be extracted along with caffeine in the aqueous. Hence, pure caffeine is preferentially to be extracted through liquid-liquid extraction. Figure 1 shows the molecular structure of caffeine.
Figure 1 Structure of caffeine or known as 1,3,7-trimethyl-2,6-purinedione
Apparatus:
Erlenmeyer flask, filter paper, separatory funnel, Hirsch funnel
Materials:
Tea leaves, calcium carbonate, cotton wool, magnesium sulphate anhydrous, petroleum ether, dichloromethane, sodium chloride
Instruments:
IR spectroscopy, gas chromatography-mass spectrometer
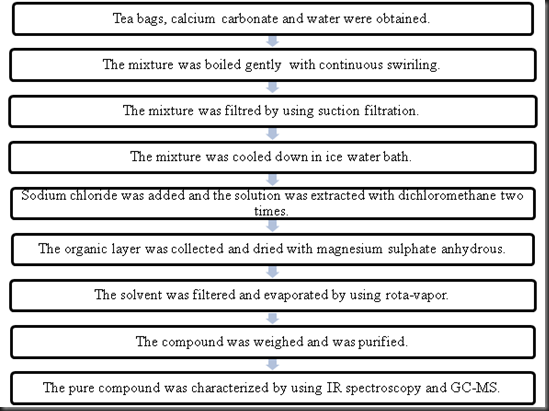
Procedures:
Isolation of caffeine and Purification of Caffeine by Sublimation
Results and calculations:
Table 1: Weight of crude caffeine
| Weight of teas | 20.0108g |
| Weight of empty round bottom flask | 113.2919g |
| Weight of (round bottom flask + crude caffeine) | 113.4313g |
| Weight of crude caffeine | 0.1394g |
Percentage yield = Mass of crude caffeine / mass of tea leaves x 100%
= 0.1394g / 20.0108g x 100%
= 0.70%
Table 2: Weight of pure caffeine
| Weight of filter paper | 0.7936g |
| Weight of petri dish | 49.9900g |
| Weight of filter paper + petri dish + caffeine | 50.7857g |
| Weight of pure caffeine | 0.0021g |
Recovery percentage of pure caffeine
= Mass of purified caffeine / mass of crude caffeine x 100%
= 0.0021g/ 0.1394g x 100%
= 1.51%
Table 3: Comparison of significant stretches between and standard caffeine and isolated caffeine
| Sources | Standard caffeine | Isolated caffeine | Journal (Paradkar & Irudayaraj, n.d.) |
| Significant stretches | Wavenumber, v (cm-1) | ||
| C=O stretch | 1701 | 1701 | 1705 |
| C=C stretch | 1663 | 1663 | 1659 |
| C=N stretch | 1546 | - | 1596 |
| C-N stretch | 1357 | - | 1369 |
Discussion:
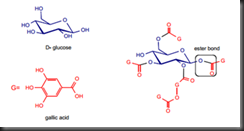
Low molecular weight of tannin is soluble in organic solvent and it makes isolation of caffeine more difficult. Tannin contains hydroxyl group that can form ester bond with gallic acid in which the ester bond can be easily cleaved. Figure 2 shows the ester bond between tannin and gallic acid.
Figure 2 Structure of hydrolysable tannin.
Initially, some calcium carbonate was added into the tea leave and then the mixture was boiled in the water during solid-liquid extraction. Calcium carbonate was used to hydrolyze tannin to produce glucose and calcium salt of gallic acid in which they are not soluble in organic layer due to their high polarity. At the same time, the base also converted caffeine to a free base which is more soluble in organic layer.
In liquid-liquid extraction, methylene chloride (dichloromethane) was used as the organic solvent to isolate caffeine from the aqueous layer. Methylene chloride (1.33 g/cm3) is denser than water so it appears at the lower layer while upper layer is aqueous layer. Magnesium sulphate anhydrous, a drying agent was used to remove all the water molecules that possible present in the organic layer. Rota-vapor was used to evaporate all the solvent and the crude caffeine was collected.
Crude caffeine was purified by using sublimation in order to isolate a pure caffeine compound. The sublimated caffeine was cooled down and formed crystal compound on the filter paper.
From the infrared spectrum of standard caffeine, the significant stretches are 1701cm-1 (C=O stretch), 1663cm-1 (C=C stretch), 1546cm-1 (C=N stretch) and 1357cm-1 (C-N stretch). Compared to the spectrum of isolated caffeine, only two significant peaks are shown which is 1663cm-1 (C=C stretch) and 1701cm-1 (C=O stretch). This might be due to the intensity of isolated caffeine is very low.
Precaution steps:
1. Methylene chloride is toxic and a possible carcinogen. Minimize the exposure to its vapors by using it in fume hood.
2. Do not use separatory funnel point to anybody when releasing the vapour.